Iodine-131 Thyroid Therapy
Background
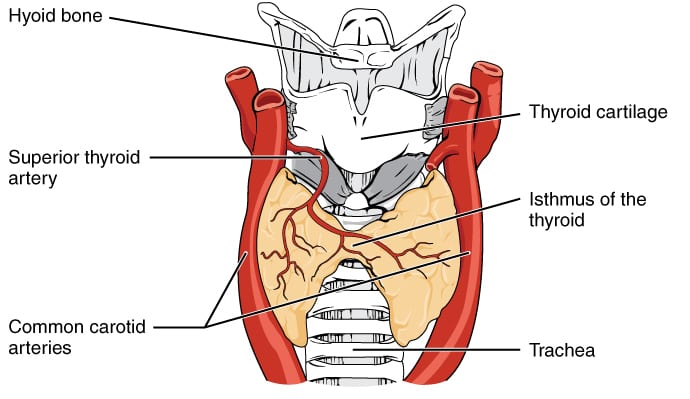
Thyroid Anatomy
The thyroid is an endocrine gland located in the front of the neck inferior to the laryngeal prominence (Adam’s apple). Thyroid cells are also the only cells in the body able to absorb iodine which is converted to thyroid hormones. Several bodily functions are regulated by thyroid hormones including heart rate, blood pressure, body temperature, and weight.
Thyroid Cancer
There are four types of thyroid cancer listed below from most to least common.
- Papillary – 86% of thyroid cancers. Slow growing and curable.
- Follicular – 9% of thyroid cancers. Also slow growing and curable.
- Medullary – 2% of thyroid cancers. This cancer can great but is treatable if caught early. Hereditary to some degree.
- Anaplastic – 1% of thyroid cancers. Fast growing and aggressive. Often difficult to control.
Symptoms
While some patients may have no symptoms others may experience the following symptoms:
- Lump in neck
- Coughing
- Difficulty swallowing
- Vocal changes
Risk Factors
- Most common in population over 30
- More common in women than men
- 45,000 women and 15,000 men diagnosed with thyroid cancer in 2013
- Radiation exposure
Diagnosis
Diagnosis is based upon several tests including:
- Blood tests for thyroid-stimulating hormone (TSH)
- Ultrasound to confirm presence of a nodule or microcalcifications
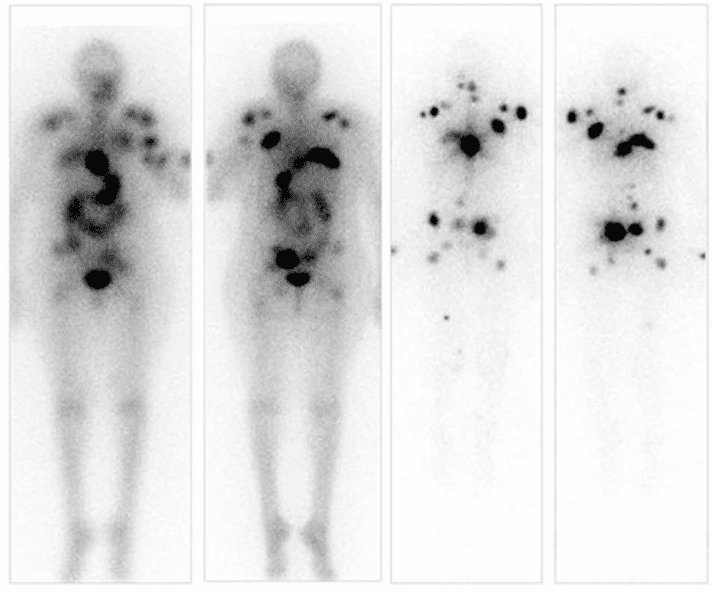
- Thyroid scan assessing iodine uptake
- I-123 SPECT imaging may be used to assess thyroid function and disease spread
- Biopsy may be taken
Treatment Options
- Thyroidectomy
- Hormone treatment
- I-131 therapy
Radioactive Iodine-131 Therapy
Radioactive iodine therapy typically follows thyroidectomy meaning which leaves very little thyroid tissue intact. Absolute contradictions include pregnant patients and patients currently breast feeding. Breast feeding should have stopped 6 months prior to administration because of increased uptake in the breast when lactating.
I-131 Quick Facts
| Nuclide | Half-life | Decay mode | Energy (beta particle) | Thyroid Administered Activity |
|---|---|---|---|---|
| I-131 | 8 days | Beta- | 180keV | 30-300mCi |
Prescription and Administration
30-300mCi is administered orally in the form of a liquid or pill. There is some evidence that lower administered activity may be as effective as higher activity administrations.
Administration Procedure
- Administration should be performed in a private room with a posted “Radioactive Materials” sign.
- The I-131 should remain in the shielded contained until administration.
- Following administration, all people and items leaving the treatment room should be surveyed to assure there has been no unintentional I-131 spread.
- Items contaminated with I-131 need to be cleaned or held to decay.
- Patient is usually surveyed at 1m to determine if they may be released.
- Once the room is clear it is also surveyed and released for its next use.
Administration Room and In-patient Room Preparation
Patient room preparation is guided by NRC 10 CFR 35.315 (external link). Typical precautions include:
- Room should be private with a private sanitary facility.
- “Radioactive Materials” sign must be visibly posted.
- A chart on the door must indicate how long visitors may stay in room.
- Covering all surfaces with disposable materials.
- Preventing housekeeping and other staff from entering.
- All food service items (plates, utensils, etc) should be disposable.
- Visitors should be discouraged.
- Pregnant visitors and minors should be prohibited.
- No items should leave the room without being surveyed.
- Radiation safety officer must be notified in event of medical emergency or death of patient.
Key point: Although the beta decay electrons are responsible for delivering most of the thyroid dose, photons released as Xe-131 returns to its ground state are responsible for most dose to the public.
Patient Release
In the U.S.A. release of patients administered I-131 is governed by the nuclear regulatory commission 10 CFR 35.75 (external link) which states the following that an individual may be released if that individual will not cause any other individual to receive more than 5mSv. Additionally, written instructions on safety precautions must be provided if the released individual may cause an exposure of greater than 1mSv.
NRC Regulatory Guide 8.39
NRC Regulatory Guide 8.39 – Release of Patients Administered Radioactive Materials (External Link)
The NRC has extended regulatory guidance for release of patients administered radioactive materials based on three criteria listed below. Patient may be released when any one of these criteria are met.
- Administered Activity
- Patient released if administered less than 33mCi (1.2GBq) for I-131.
- Written instructions not required if administered activity is less than 7mCi (0.24GBq) for I-131.
- Administered Dose Rate
- Patient released if dose rate measured at 1 meter from patient is equal to or less than 0.07mSv/hr (7mrem/hr).
- Written instruction not required if dose rate measured at 1 meter from patient is equal to or less than 0.02mSv/hr(2mrem/hr).
- Patient-Specific Dose Calculations
- May perform a patient specific calculation accounting for effective half-life, patient shielding, and other factors. If this calculation predicts less than 5mSv (0.5rem) max dose to another member of the public, the patient may be released.
- Methodology is found in NRC 10 CFR 35.75(c) (external link).
Key point: Agreement states may have release criteria that is more strict than the criteria noted above.
Inpatient Treatments
Patients may be likely to exceed the allowable release criteria due to the following:
- High administered activity (>200mCi)
- High density housing situations
- Shared rooms especially with children
- Shared bathrooms
- Cognitive and psychiatric limitations
Travel limitations (need for long bus/plane/train rides to and from treatment)
Navigation
Not a Premium Member?
Sign up today to get access to hundreds of ABR style practice questions.