Radioactive Decay Modes
Nuclide Relationships
Isotopes
Atoms having the same number of protons (Z) but different number of neutrons (N) are isotopes.
Isobars
Atoms having the same mass number (A) are isobars.
Isotones
Atoms having the same number of neutrons (N) but a different number of protons (Z) are isotones.
Isomers
Atoms of the same element (same Z and N) but which are in different excited states are isomers.
Beta Decay
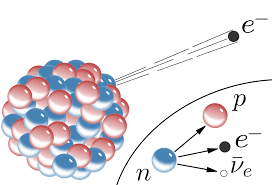
Beta- (Electron) Decay
Beta- (β-) decay converts a neutron into a proton, an electron, and an anti-neutrino.
![]()
- Occurs in radionuclides with high neutron-to-proton ratio (i.e. lying above the line of stability)
- A neutron is converted to a proton, the electron is required to conserve charge and the anti-neutrino is needed to conserve Lepton number
- Continuous energy spectrum
- Energy shared between electron and anti-neutrino
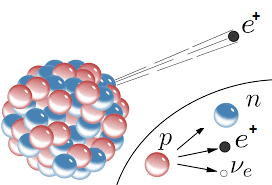
Beta+ (Positron) Decay
Beta+ (β+) decay converts a proton into a neutron, a positron (anti-electron), and a neutrino.
![]()
- Occurs in radionuclides with low neutron-to-proton ratio (i.e. lying below the line of stability)
- A proton is converted to a neutron, the positron is required to conserve charge and the neutrino is needed to conserve Lepton number
- Continuous energy spectrum
- Energy shared between positron and neutrino
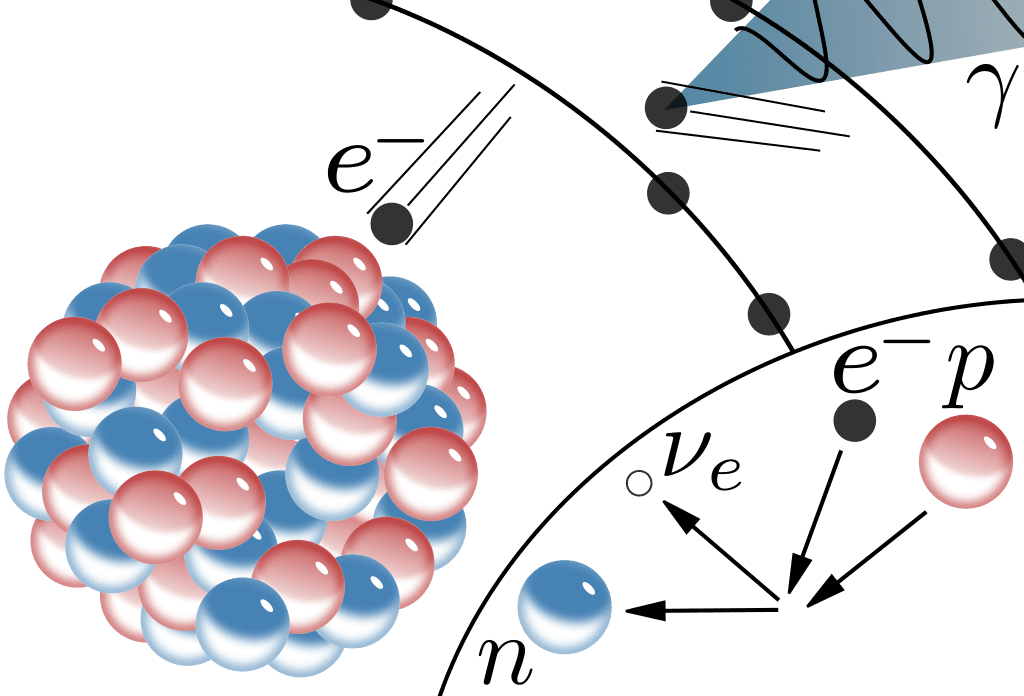
Electron Capture
During electron capture, an electron is captured by the nucleus which converts a proton into a neutron.
![]()
- Occurs in radionuclides with low neutron-to-proton ratio (i.e. lying below the line of stability)
- The captured electron is usually from the inner orbital shell and leaves a vacancy.
- Energy release to fill vacancy results in emission of characteristic x-rays and/or Auger electron emission.
Key Point: Beta+ decay and Electron Capture are competing processes. That is, they are both able to convert a proton into a neutron producing isobars.
Internal Conversion
Internal conversion allows an excited nuclear state to lower its energy to a ground state via the emission of a gamma-ray or orbital electron. If an orbital electron is ejected, characteristic x-rays and/or Auger electrons may be emitted as well.
![]()
Photodisintegration

Photodisintegration occurs when a high energy photon interacts with an atomic nucleus and imparts sufficient energy to cause the nucleus to loose one or more nucleons.
![]()
- Threshold energy (minimum required energy) for photodisintegration is around 10MeV for most nuclei
- Beryllium and deuterium are notable exceptions with threshold energies around 2MeV
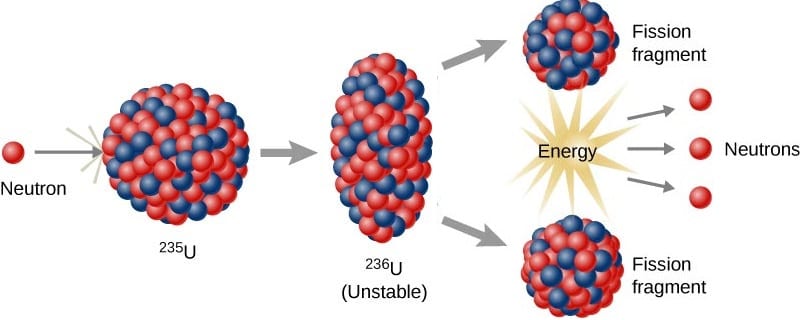
Nuclear Fission
Fission is the term for nuclear disintegration of high Z nuclei.
![]()
- May be spontaneous or induced by neutron bombardment
- In neutron induced fission, the neutron is absorbed by the nucleus making it unstable. The nucleus subsequently decays.
Nuclear Fusion
Fusion is the process of combining two low atomic number elements into a larger atomic number element.
![]()
Common fusion reactions include:
Deuterium/Deuterium Fusion
![]()
Deuterium/Tritium Fusion
![]()
Deuterium/Helium Fusion
![]()

Navigation
Not a Member?
Sign up today to get access to hundreds of ABR style practice questions.