Hepatic Brachytherapy (Y90 Microspheres)
Selected Readings
AAPM TG-144: Dosimetry, Imaging, and Quality Assurance Procedures for 90Y Microsphere Brachytherapy (external link)
Overview
Ytterium-90 microspheres are used for the treatment of Hepatocelular Carcinoma and metastatic tumors of the liver.
90Y incorparated into glass or resin sphere approximately 20-60μm in diameter. The spheres are injected into the hepatic artery, often accessed through the femoral artery. This approach to delivery is very effective because the hepatic artery supplies 80-100% of the blood flow to these tumors. In contrast, the unaffected liver will only receive 20-30% of its blood from the hepatic artery and the remaining 70-80% from the portal vein. The use of Y-90 microspheres allows for greater tumor dosing compared to external beam which may be limited by tissue toxicity to non-tumor destroying levels.
There are currently two types of Yttrium-90 microspheres available in the US. SIR-Spheres uses a resin based material and is available in 3GBq vials. TheraSphere uses a glass based material and is available in 3, 5, 7, 10, 15, and 20GBq vials. Theraspheres are slightly denser (3.29 vs 1.6 g/cm3) and has a higher activity per sphere (2500 vs 50 Bq).
Prescriptions
Prescriptions may be written either in either terms of administered activity or mean dose to the target liver lobes. Mean target lobe doses are commonly in the range of 120-150Gy!
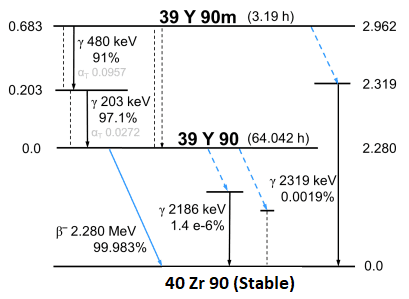
Yttrium-90
Yttrium-90 (90Y) is a beta (electron) emitter with an average energy of 0.9267 MeV and a half-life of 2.67 days. Over 90% of emitted energy is absorbed within 5.3mm and the maximum range of emitted electons is 11mm. Y-90 is most commonly used in radiation therapy as the source in microsphere liver treatments.
Quick facts
- Half life: 2.67 days
- Decays approximately 1% per hour
- Decay mode: β–
- Beta energy: 0.9267 MeV (mean)
Determining Patient Eligibility
Imaging Studies
Triple-phase contrast enhanced CT is common to determine liver volume and extent of tumor burden.
MRI, especially with Gadolinium contrast, may also be used to determine volumes.
PET imaging using 18F-FDG is commonly used to assess regions of metabolically active tumor.
SPECT/Gamma Camera imaging is useful in determining the expected distributions of microspheres. This is accomplished using 2-4mCi of 99mTc microaggregated albumin (MAA) infused into the liver.
Key Point: SPECT imaging of the Y-90 bremsstrahlung is also used post procedure to verify microsphere deposition.
An angiographic procedure is performed prior to treatment to asses blood flow and to guide the catheter through the femoral artery to the hepatic artery and into treatment position
Key Point: Lung Shunting
The 99mTc microaggregated albumin (MAA) imaging is used to asses the fraction of microspheres expected to enter (be shunted to) the lungs. If lung shunting is >20% of administered activity, the patient is a poor candidate for microsphere therapy.
Indications and Contraindications
Data from Table II TG-144
| Indications | Contraindications |
|---|---|
| Unresectable hepatic primary or metastatic disease | Limited hepatic reserve |
| Liver dominant tumor | Estimated lung dose greater than 30Gy |
| Life expectancy greater than 3 months | Uncorrectable extrahepatic deposition |
Dosimetry
Medical Internal Radiation Dose (MIRD) Committee of the Society of Nuclear Medicine Standard
- k is a constant yielding dose rate in the desired units
- E¯ is the average energy emitted per nuclear transition
- A is the activity
- m is the mass of tissue absorbing the radiation
- For the target, this is often taken as the mass of the affected lobes of the liver.
- The the lungs, this is often taken as the total lung mass.
- Important: Remember to subtract the fraction of activity shunted to the lungs from the liver activity and vice-versa during calculation.
Image Based Dosimetry
The 99mTc MAA study data may be used prospectively to determine the expected dose distribution to the patient. This is accomplished using convolution of the activity distribution and a Monte Carlo generated dose deposition kernel. The dose deposition kernel is spatially invariant meaning.
Activity Calibration
Key Point: The is no current standard method of determining the source activity independent of the manufacturers measurements. Both SIR-spheres and TheraSpheres provide end-users with calibration samples with which they can determine their own calibration coefficients for their equipment.
NIST uses liquid scintillation to characterize Y-90 in solution. This procedure is very accurate and is a primary measurement (i.e. relies on fundamental principles rather than calibration factors).
TheraSphere calibration is NIST traceable through the NIST Radioactivity Measurement Assurance Program (NRMAP).
SIR-spheres are not NIST traceable. Instead they are calibrated against the Australian Nuclear Science and Technology Organization (ANSTO) and the Australian Radiopharmaceuticals and Industrials (ARI) standards. These methods dissolve the SIR-spheres into a solution and measure the activity using an ionization chamber. This procedure induces additional error (relative to direct liquid scintillation) as it is dependent upon the volume and homogeneity of the sample. Users are supplied with calibration samples which allow them to determine calibration factors for their own equipment.
Many end-user choose to trust the manufacturer’s calibration value of treatment sources but it remains the responsibility of the institution to verify this calibration. Agreement to within 5% is considered acceptable. Deviations exceeding 10% should be reported to the manufacturer.
Regulatory Requirements
The US Nuclear Regulator Commission (NRC) regulated 90Y microspheres as medical devices under 10 CFR part 35 code.
Navigation
Not a Premium Member?
Sign up today to get access to hundreds of ABR style practice questions.