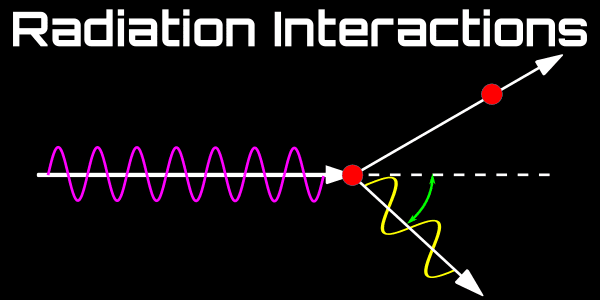
Introduction to Radiation
What is Radiation?
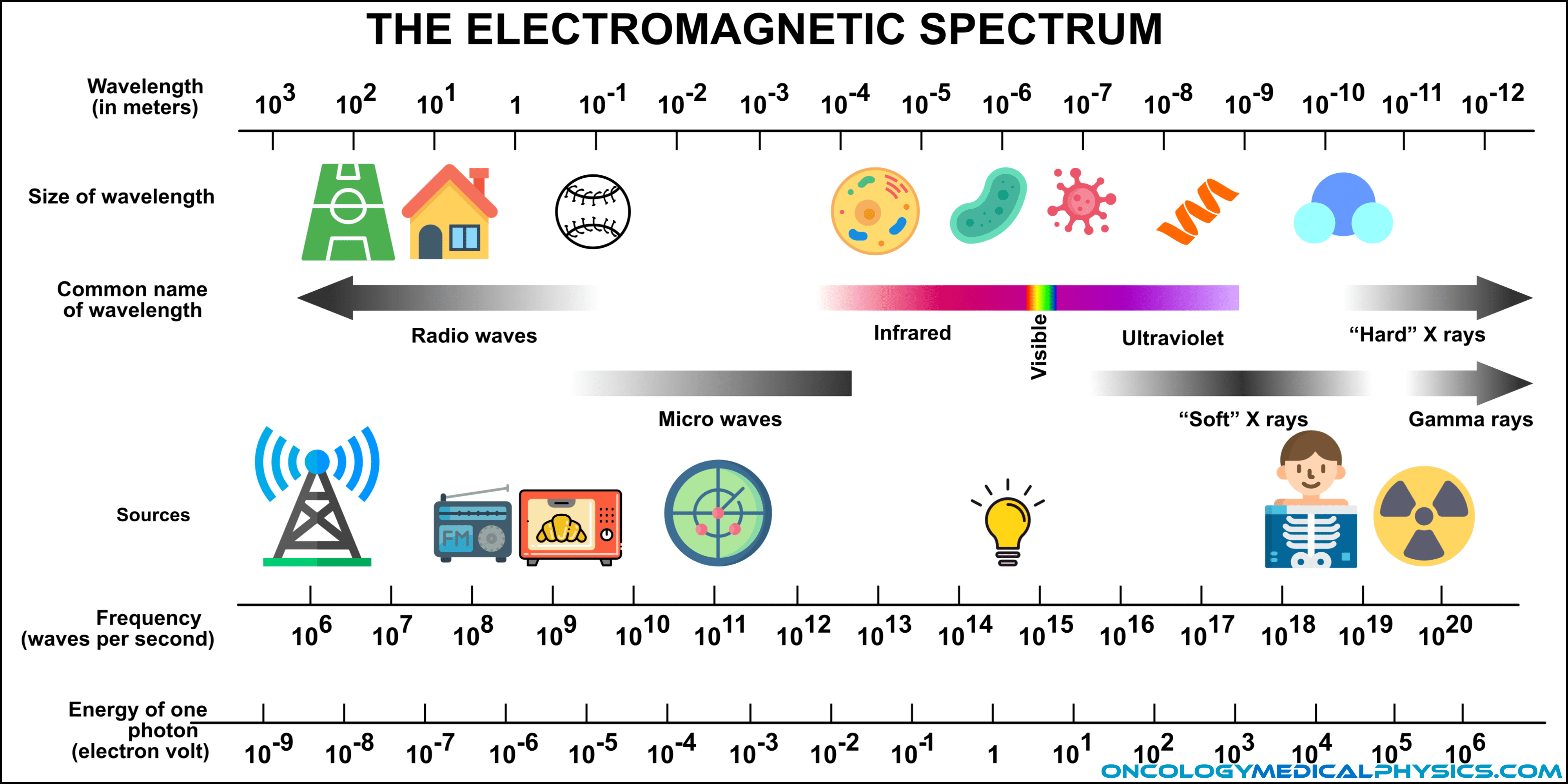
Radiation is the transmission of energy via waves or particles.
Radiation may be the result of natural sources (Uranium-223 decay, solar emissions, cosmic rays, etc.) or may be man-made (an X-ray machine, linear accelerators, an LED bulb, etc.).
How is Radiation Classified?
Because of the variety of radiation sources and properties, they are often categorized by energy (ionizing vs non-ionizing), type (electromagnetic vs. particle) or charge (charged or uncharged).
What is Ionizing Radiation?
Ionizing radiation is radiation that carries sufficient energy to detach electron from atoms or molecules.
- Ionizing radiation is potentially hazardous because of its ability to damage DNA.
- The energy threshold to be considered ionizing radiation is between 10eV and 33eV for photons.
- This is in the ultraviolet region and explains why sun exposure causes sunburn (erythema)!
Key Point: When people (especially medical physicists) are discussing radiation, they are usually discussing highly energetic ionizing forms of radiation.
Types of Radiation
Electromagnetic Radiation (Photons)
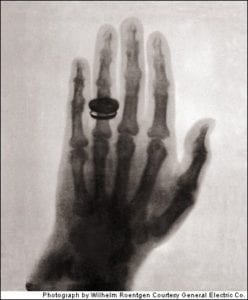
X-rays
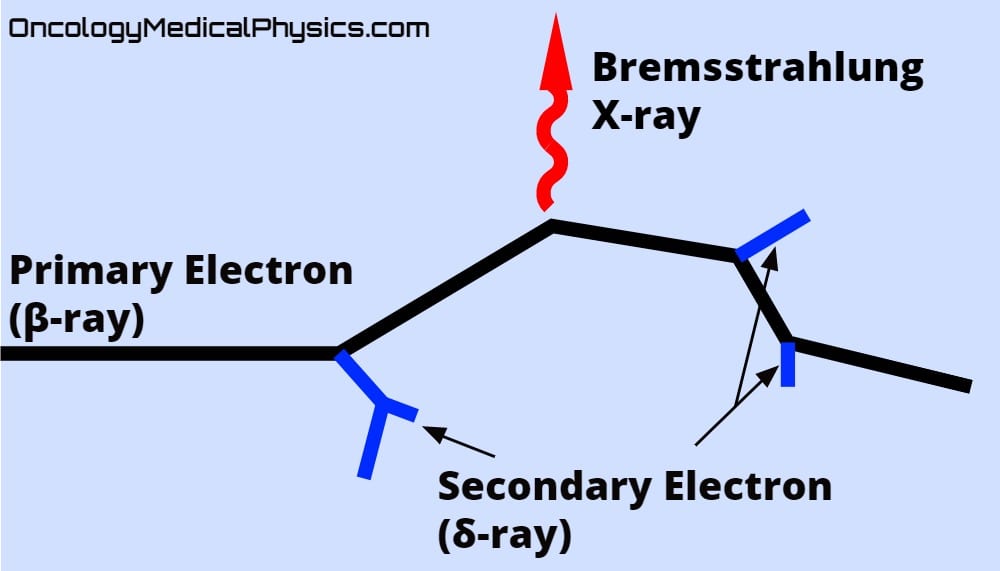
X-rays are photons with an energy between about 10eV and 100keV. They may be produced naturally as electrons transition from high to low energy orbitals (characteristic X-ray emission) or as a result of a Bremsstrahlung interaction between an electron and a nucleus.
Classification
- Uncharged
- Electromagnetic
- Ionizing
Gamma Rays
Gamma (γ) rays are highly penetrating electromagnetic radiation arising from the decay of radioactive atomic nuclei. The term “gamma ray” may be used to refer to any photon with an energy about 100keV, including those generated in a linear accelerator.
Classification
- Uncharged
- Electromagnetic
- Ionizing
Cherenkov Radiation
Electromagnetic radiation emitted when a charged particle passes through a dielectric medium at a speed greater than the speed of light in that medium. As a result of the high speed, the particle is forced to rapidly decelerate releasing a photon. Cherenkov radiation is responsible for the characteristic blue glow of an underwater nuclear reactor. While seriously cool, Cherenkov radiation is generally non-ionizing and is not used for clinical treatments.
Classification
- Uncharged
- Electromagnetic
- Non-ionizing
Particle Radiation
Alpha Particles
Alpha particles consist of a helium-4 nucleus (two protons and two neutrons). Alpha particles are produced primarily through alpha decay of heavy elements. They are charged (2+) ionizing radiation. Because of their large charge and mass, they are considered a high LET form of radiation. This increases their biologically effective dose by a factor of 10-20 relative to photons or electrons.
Classification
- Charged
- Particle (Helium ions)
- Ionizing
Additional Information
- Typical energy: 5MeV
- At this energy, alphas are non-relativistic traveling at only about 0.05c.
- Range in air of about 4cm
- Range in tissue 40-90um
- RBE: 10-20
- LET: 100keV/um (High)
- Charge: 2+
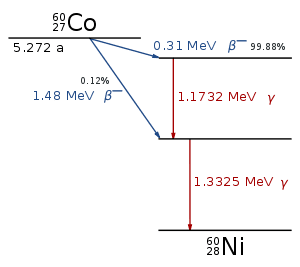
Beta Particles
Beta particles are energetic electrons or positrons (anti-matter electrons with a positive charge) that are emitted by radioactive decay of an atomic nucleus. Electron beams may also be generated using a heated cathode (a process known as “boiling off”) and accelerated via an electric field, as in a linear accelerator.
Classification
- Charged
- Particle (Electrons)
- Ionizing
Additional Information
- Naturally occurring beta particles have continuous energy distribution due to beta decay neutrino.
- Charge: 1- (electrons), 1+ (positrons)
- RBE: 1
Delta Particles
Delta (δ) particles are secondary electrons liberated from a medium by interaction with a primary electron.
Delta rays which are created by a delta ray may be referred to as an "epsilon ray," although this notation is not in common use in medical physics.
Classification
- Charged
- Particle (Electrons)
- Ionizing
Proton Radiation
Protons are the heavy, positively charged constituent of an atoms nucleus. Although proton radiation is a byproduct of several nuclear reactions (e.g. 14N + α→ 17O + p) and even lightning strikes, it is also routinely produced via cyclotrons and used in proton therapy and particle physics. Although more expensive to produce clinically than electron or photon beams, they have a sharp Bragg peak and are able to deposit large doses locally while sparing surrounding tissues. This makes proton therapy a good choice for several situations including pediatric radiation therapy.
Classification
- Charged
- Particle (Protons)
- Ionizing
Additional Information
- Charge: 1+
- RBE: 1.1
Neutron Radiation
Neutron radiation consists of energetic free neutrons. Neutrons are released as the result of neutron fission or fusion or from radioactive decay. Interestingly, free neutrons are unstable (mean life = 887s) and will decay into a proton, an electron, and an anti-electron-neutrino. Because neutrons have no charge, they cannot interact with matter through electric fields and thus have a significantly longer range than charged particles. Neutrons are typically divided into “fast” and “thermal” (slow) categories because of differences in their properties at these ranges. Fast neutrons are best attenuated by kinetic impact with hydrogen-rich materials such as plastics. Due to conservation of energy and momentum, hydrogen's mass (which is near that of a neutron) allows it to absorb the maximum amount of energy in a collision with a neutron.
Once the neutron has energy of less than about 0.025eV, it is considered a thermal neutron (so named because this is the energy range of kinetic energy in most materials). The neutron absorption cross-section is increased greatly at thermal energies in certain elements such as Boron-10. Hence Boron’s use in high energy linear accelerator shielding.
Classification
- Uncharged
- Particle (Neutron)
- Ionizing
Additional Information
- Charge: 0
- RBE immediate radiation injury: 1
- RBE cataracts, leukemia, and genetic changes: 4-10
Navigation
Not a Member?
Sign up today to get access to hundreds of ABR style practice questions.