Radium-226
Overview
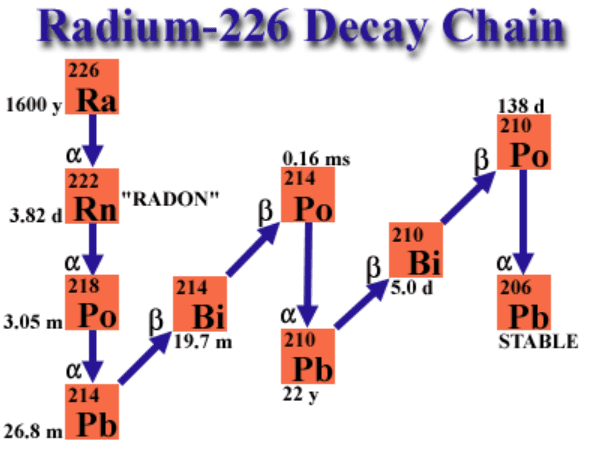
Radium-226 (226Ra) is was the first common radionuclide used in brachytherapy since it’s discovery in 1898. With a half-life of 1600 year, Ra-226 is the most stable form of Radium. Radium undergoes alpha decay to Radon-226. The decay chain of Radium 226 ends in stable lead (Pb-206). Approximately 49 gamma rays with energies ranging from 0.184 to 2.45 MeV are released during the decay from Radium to Lead.
Because Radium-226 has a significantly longer half life than it’s daughter products, it will exist in secular equilibrium with it’s daughter products. The time to reach equilibrium for a new source is approximately 1 month. Finally the decay process of Ra-226 results in a buildup of gas Radon gas. This can be an important consideration in the design of an encapsulated Radium brachytherapy needle.
\begin{equation} ^{226}_{88}Ra \longrightarrow ^{222}_{86}Rn + ^{4}_{2}He \end{equation}
Quick Facts
- Half life: 1,600 years
- Decay Mode: Alpha, Beta
- Average Photon Energy: 0.83 MeV (approximately 49 photons released per decay with an energy range of 0.047 – 2.45 MeV)
- Exposure Rate Constant:
- \( \Gamma_{\delta} = 8.25 \frac{R \ cm^2}{mCi \ hr} \)