Iridium-192
Overview
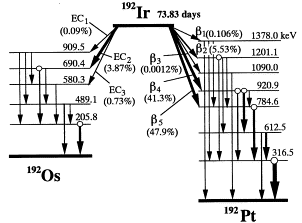
Iridium-192 (\(^{192}Ir\)) is the most common isotope used in high dose rate (HDR) brachytherapy. It is a man-made isotope produced by neutron bombardment of Iridium-191. \(^{192}Ir\) decays primarily through \(\beta ^{-}\) decay to an excited state of Platinum-192 which is stable. Approximately 5% of decays occur via electron capture yielding an excited state of Osmium-192 which is also stable. It is regarded as superior to cobalt-60 and cesium-137 due to it’s higher specific activity which allows for smaller source sizes. One disadvantage to \(^{192}Ir\) is it’s short half life (73.83 days) which results in a loss of activity of approximately 1% per day. This necessitates source changes every 3-4 months.
Quick Facts
- Half life: 73.83 days
- Activity loss per day (quick estimate): 1%/day
- Decay modes
- 95.6% \(\beta ^{-}\) decay to excited state of \(^{192}Pt\)
- 4.4% Electron capture to excited states of \(^{192}Os\)
- Exposure rate constant1
- \((\Gamma_{\delta})_\chi = 4.69\frac{R \ cm^2}{mCi-h}\)
- Air Kerma Rate Constant
- \((\Gamma_{\delta})_\kappa = 4.11 \ \frac{cGy \ cm^2 }{mCi \ h}\)
- Photon Energy
- 0.136-1.06 (0.38 average) MeV
- Half-value layer (mm lead): 2.5mm
Works Cited
- 1.Nath R, Anderson L L, Luxton G, Weaver K A, Williamson J F, Meigooni A S. Dosimetry of Interstitial Brachytherapy Sources: Recommendations of the AAPM Radiation Therapy Committee Task Group No. 43. Medical Physics; 1995:209-234.